Life Sciences
Offshore Trial and Error

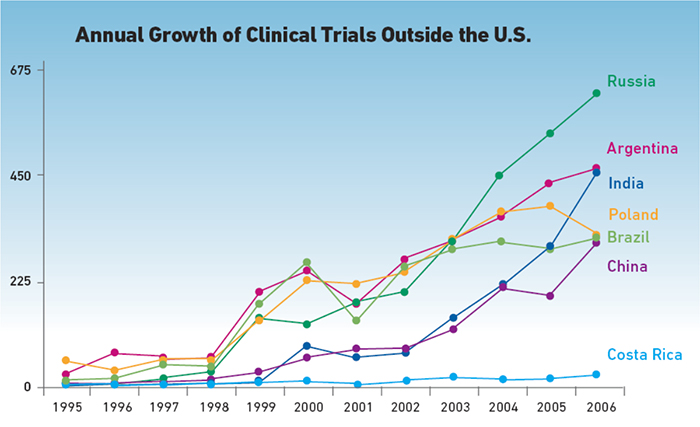
American life sciences companies conducting clinical trials abroad confront a tangle of risks that require careful planning, well-drafted documents and the aid of local organizations to navigate laws, regulations and customs of each test site, lest errors and oversights produce costly delays and faulty clinical data.
The biggest risk for overseas clinical trials, said Jim Walters, managing director, Aon Risk Solutions’ life sciences group, is “working through the myriad regulations,” both for insurance and clinical requirements, so “sponsors can meet local ethics committees and regulatory requirements to get the trial started.”
American companies’ efforts to grapple with compliance risk, said Jack Bodden, managing director, global risk management, Marsh, is complicated by different regulatory requirements in each country.
Compensation requirements for participants in clinical trials vary broadly between countries, he said, and the “ask” of sponsor companies from regions and clinical sites often differ from the country’s written regulations. “Not getting regulatory risk managed well can delay or halt the trial,” he said.
For example, the United States does not require clinical trial liability insurance coverage — the equivalent of product liability insurance for clinical trials. But as a practical matter, the ethics committees of the hospitals where trials take place would reject any trial that did not show evidence of the sponsor’s insurance, said Frank Goudsmit, senior vice president, life sciences and food market segments, Chubb.
Likewise, accident insurance for participants’ travel to and from the trial site has become customary in Germany, although it’s not required by statute, Goudsmit said.
Until recently, said Bodden, several European Union countries required sponsors to provide evidence of insurance protection for its patient population that exceeded protections in commercially available products.
“Sponsors couldn’t comply because there wasn’t even an insurance option available to satisfy the requirement,” he said. Ultimately, pharmaceutical companies organized and negotiated a compromise with regulators that enabled their compliance while preserving the highest standards of patient safety and protection.
Local Partners
Even within a country, regional and local customs can vary, calling for experienced local partners to navigate variations in written and unwritten rules, said Dan Brettler, life science and technology co-practice leader, Conner Strong & Buckelew.
Individual clinical sites may look to expand requirements beyond clinical trials liability, he said, such as indemnification for professional liability, or they may require local admitted insurance even where the country permits nonadmitted cover.
How does a sponsor control its risk in environments where inconsistency is the only consistent factor?
“Dealing with experienced contractors and writing comprehensive contractual agreements is part of the liability equation,” Brettler said.
“Sponsors must be mindful of insurance and indemnification contractual provisions with their CROs, clinical sites and supply chain partners, especially in countries with a history of product contaminations or regulatory violations.”
CROs, or contract research organizations, are local companies that sponsors hire to help manage development and implementation of their trials. CROs should know the local statutes, customs, ethics committees and decision-makers in the sites where a trial is proposed, said Walters.
CROs should also be vetted, said Dr. Rasika Birewar, owner, sehatINDIA Healthcare Services in Mumbai.
“The CRO shouldn’t escape risk assessment because it’s local to a country. Sponsors have to do their own due diligence on the CRO because the onus is on them to comply with regulations and laws in every site’s country.”
“The sponsor needs eyes and boots on ground in addition to the CRO,” said Rob Dickey, chief financial officer of Tyme Technologies Inc., which has conducted clinical trials in North America, Europe and India.
A manager in the direct employ of the sponsor should oversee the CRO, Dickey said. “If something falls off a table in the treatment room and hits a patient’s foot, who’s responsible?”
Medical care in that case, where the injury is unrelated to the investigational product, might be a standard part of coverage in their world, he said, but not necessarily in ours. “A lot of stuff can happen when you’re not there and there’s a seven-hour time difference. You need someone to oversee that.”
As in the case with rare diseases, sponsors sometimes conduct trials all over the world to accrue a statistically valid sampling of patients with a particular disease state, said Goudsmit. That could involve two or three patients in a dozen countries — and local representation in each of those.
Good Documents
A secondary risk, almost rivaling compliance, is appropriate documents that meet regulatory standards in the right hands well before the start of the trial, said Walters.
“Sponsors don’t want wrong language in the insurance documents to delay the trial,” said Dickey. “The ethics committee in a given hospital may not meet for another six months.”
Clinical trial liability insurance, which responds to bodily injury to patients arising from the trial, is near-standard in custom if not by statute. In an industry that quantifies risk assiduously and prices its coverage accordingly, Bodden said, “clinical trial liability insurance may cover drugs and devices that have not yet proven to be safe and effective.”
Drugs undergoing clinical trials aren’t always new; approved drugs may undergo new trials for treatment of a different disease state.
In addition to clinical trial liability coverage, all trials must include informed consent documents that provide prospective participants “adequate information to allow for an informed decision about participation in the clinical investigation,” according to the FDA.
The document must be written in language understandable by laypeople, describing the study’s purpose, duration, expectations of participants and any risks exposed in the animal trials that precede the human trials.
Many CROs buy errors and omissions (E&O) insurance to protect themselves against sponsors’ allegations of financial injury arising out of negligence.
That can easily run into millions of dollars if the trial has to be repeated, Goudsmit said.
“If the clinical data isn’t acceptable to the regulatory body because of mistakes in research — if the CRO didn’t follow good clinical practices — E&O insurance would cover the cost of re-conducting the trial,” Goudsmit said.
Clinical trials can account for two-thirds of the cost of developing a new drug, said Praveen Gupta, managing director, chief executive officer, Raheja QBE General Insurance Co. Ltd., in Mumbai.
A demonstration that an investigational drug or device meets its safety and efficacy targets is the litmus test for regulatory approval. Trials can fail to meet their endpoints — generally not insurable — if they can’t demonstrate sufficient efficacy.
If the product fails because of safety concerns that manifest themselves during the trial, participants may well have suffered bodily injury.
“If a product fails to meet the regulatory endpoint because of safety concerns,” Goudsmit said, “that can absolutely result in a human clinical trial liability claim.”
In that case, he said, patients’ attorneys will ask, what did the sponsor know about possible adverse events, or what should it have known? Was the informed consent document thorough? Was it understandable?
FDA Approval: Gold Standard
Sponsors conduct their clinical trials where they can find patients with their targeted disease state or a specific DNA pool; they may go offshore to find “treatment naïve” patients to produce unambiguous clinical data; but they universally seek their regulatory endpoint — proof of safety and efficacy — and they almost universally seek FDA approval.
FDA approval is the coveted gold standard of regulatory approval both because the U.S. market is the world’s most lucrative, offering the innovator company the greatest pricing flexibility, and because most countries will also grant approval if a product meets the FDA’s high standards, Goudsmit said.
The FDA, and most other regulatory bodies, will accept data from clinical studies conducted anywhere in the world, said Walters. “Sponsors just have to adhere to the approved protocols and follow good clinical practices.”
“Countries want to protect their populations from unproven drugs and devices,” said Bodden. “And pharmas will do everything possible to keep patients safe.”